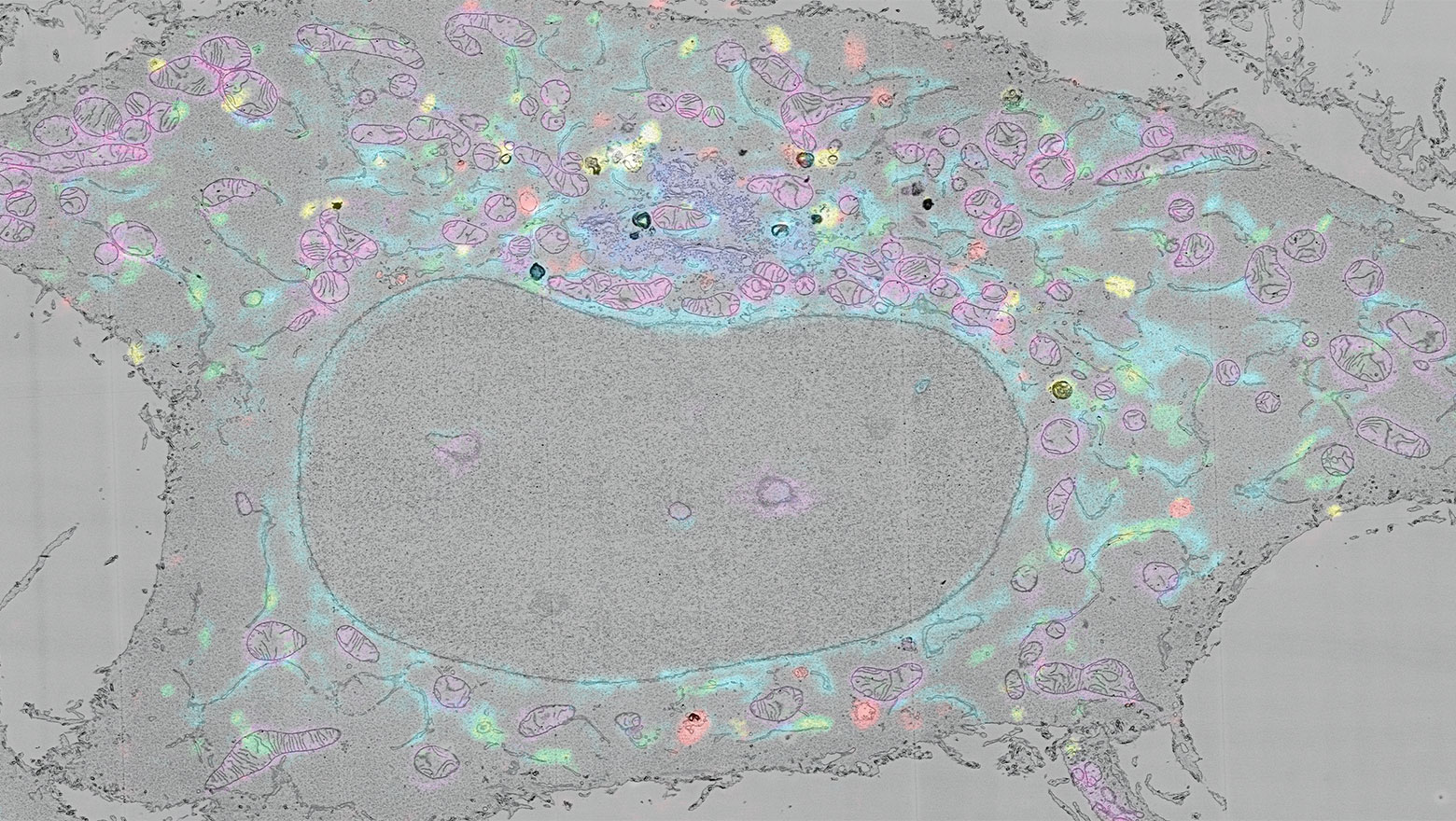
生命科学を支える 細胞生物学
News / Topics
新着お知らせ
-
CSF論文発表のお知らせ
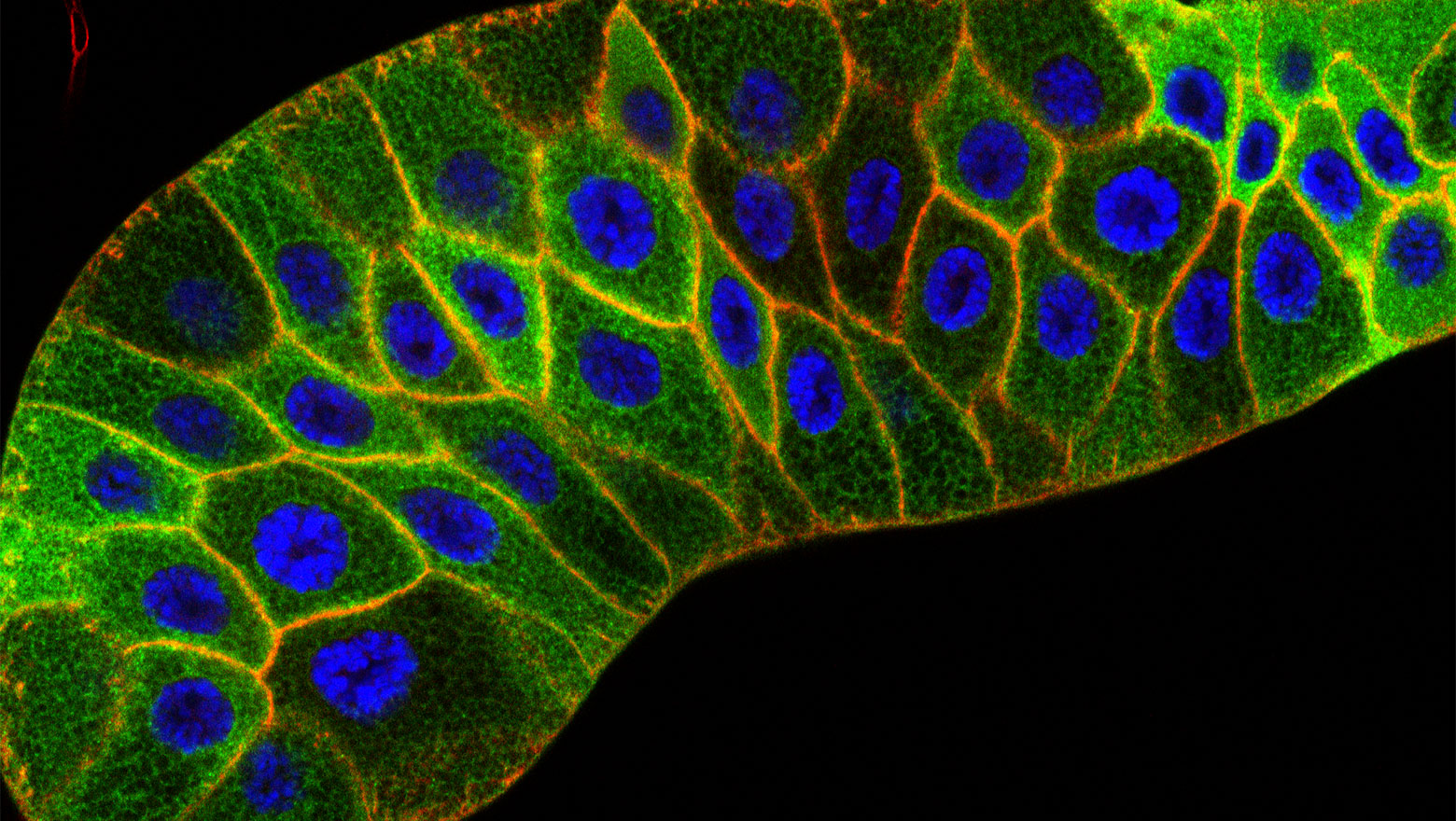
Depletion of Flot1 attenuates macropinosome-dependent mTORC1 activation in podocytes
-
CSF論文発表のお知らせ
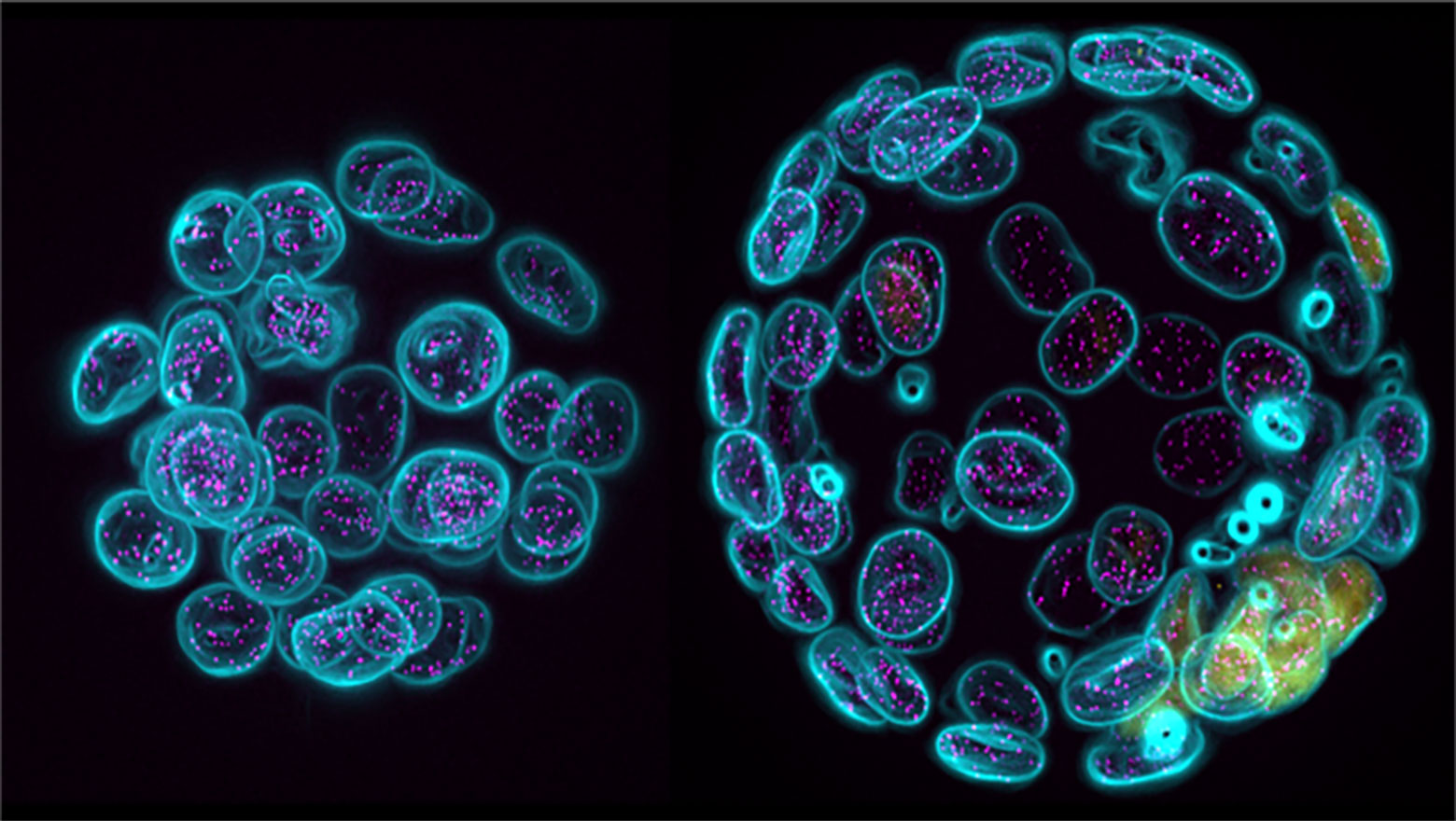
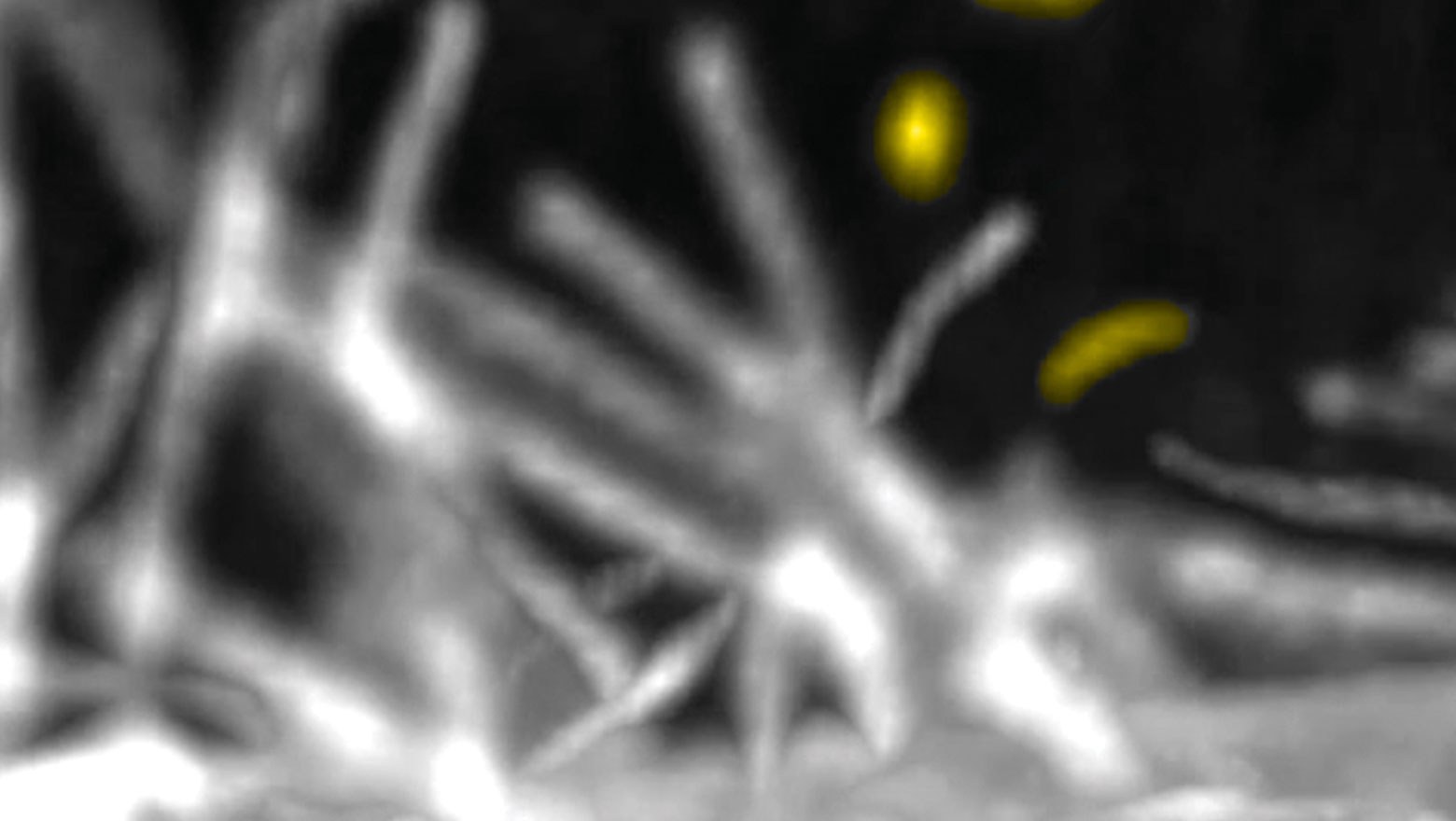
Enhanced release of ciliary extracellular vesicles suppresses cell migration and promotes cell aggregation
この論文では、孤立状態から増殖した膵がん細胞で見られる一次繊毛由来細胞外小胞の放出増加の機能的意義を解析した。
-
CSF論文発表のお知らせ
Single-nucleosome imaging uncovers biphasic chromatin dynamics in inducible human transformed cells
本研究は、ヒト細胞のがん化過程におけるクロマチンのふるまいが時間とともに再編成されることを1分子ヌクレオソームレベルで示しました。
-
CSF論文発表のお知らせ
Structural truncation of IL-1R2 enhances the anti-inflammatory activity of HeLa cells
この論文では、IL-1R2の構造欠失が可溶化を促し、IL-1デコイ機能を増強することを示しました。
-
CSF論文発表のお知らせ
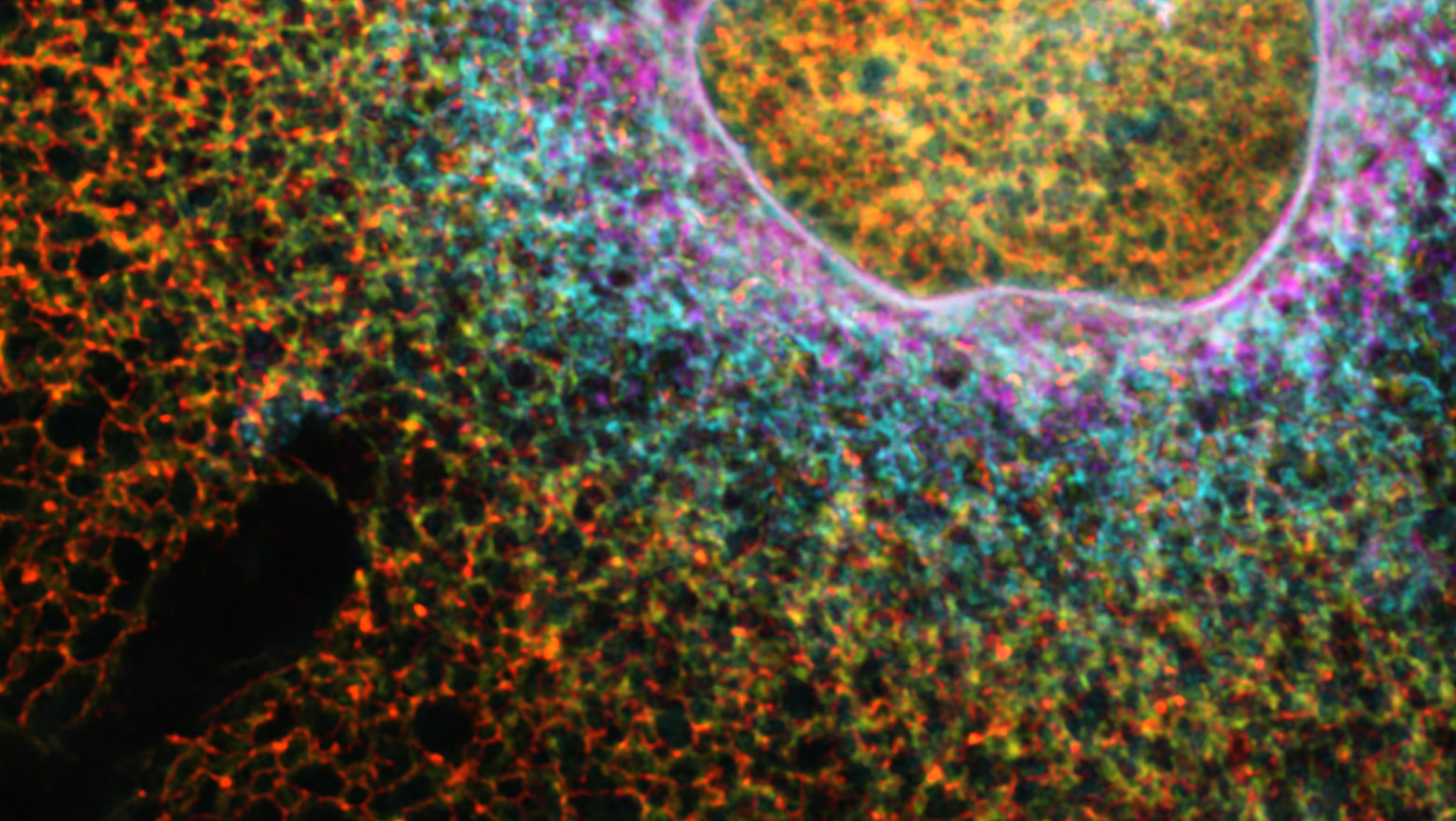
Context-dependent interactions among afadin, ZO-1, and actin filaments
この論文は、アクチン結合性足場タンパク質であるアファディンとZO-1の相互作用を、ジャンクションを持たない非上皮細胞を用いて明らかにしたものである。
Journal
会報「細胞生物」
Events
学術大会・イベント
About
当学会について