生命科学を支える 細胞生物学
News / Topics
新着お知らせ
-
CSF論文発表のお知らせ
A quantitative method to monitor STING degradation with dual-luciferase reporters
-
CSF論文発表のお知らせ
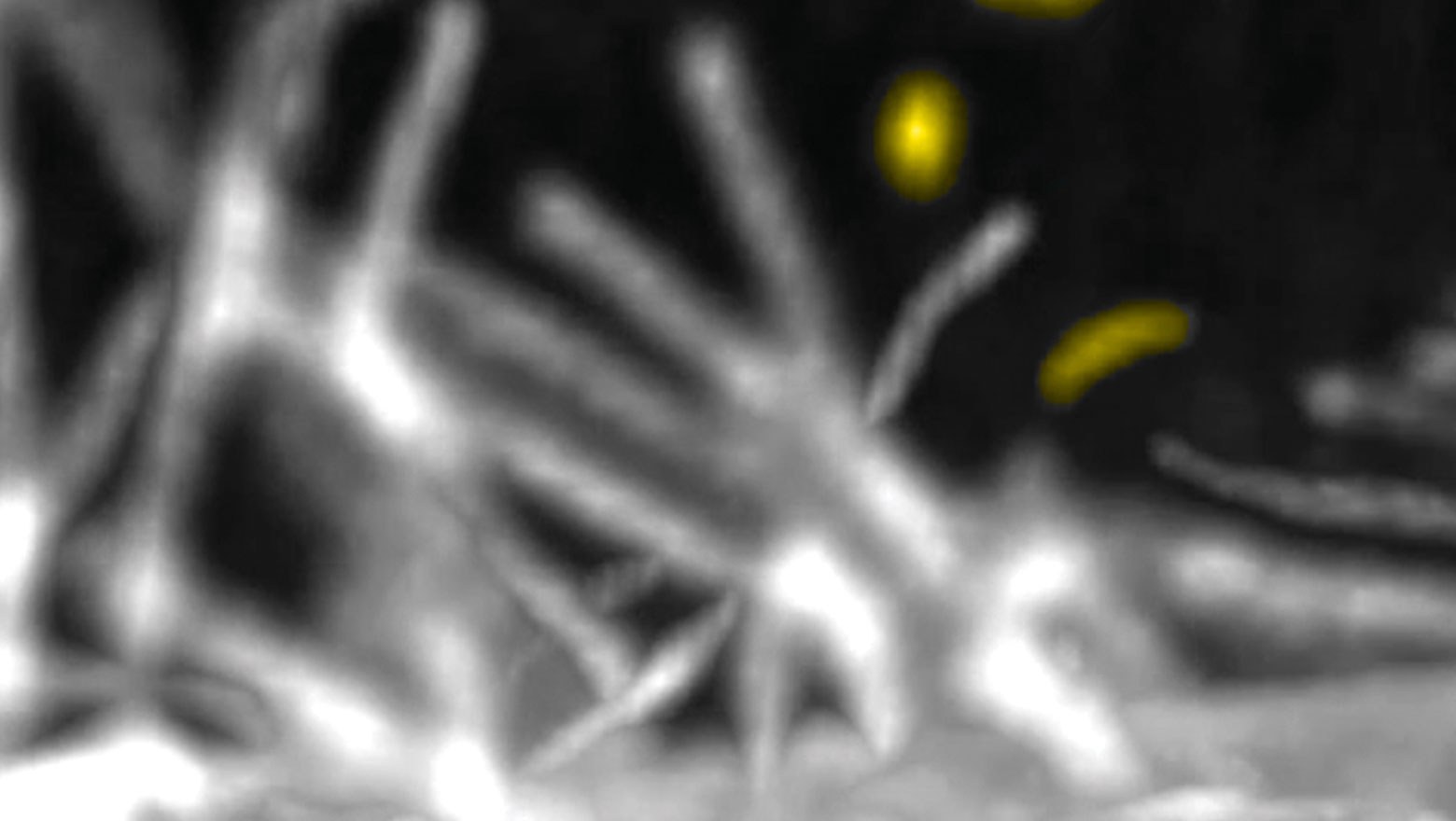
Chemokine induces phase transition from non-directional to directional migration during angiogenesis
この論文では、脳の毛細血管網形成において新生血管が標的血管へ向かって移動する機構を報告しています。
-
CSF論文発表のお知らせ
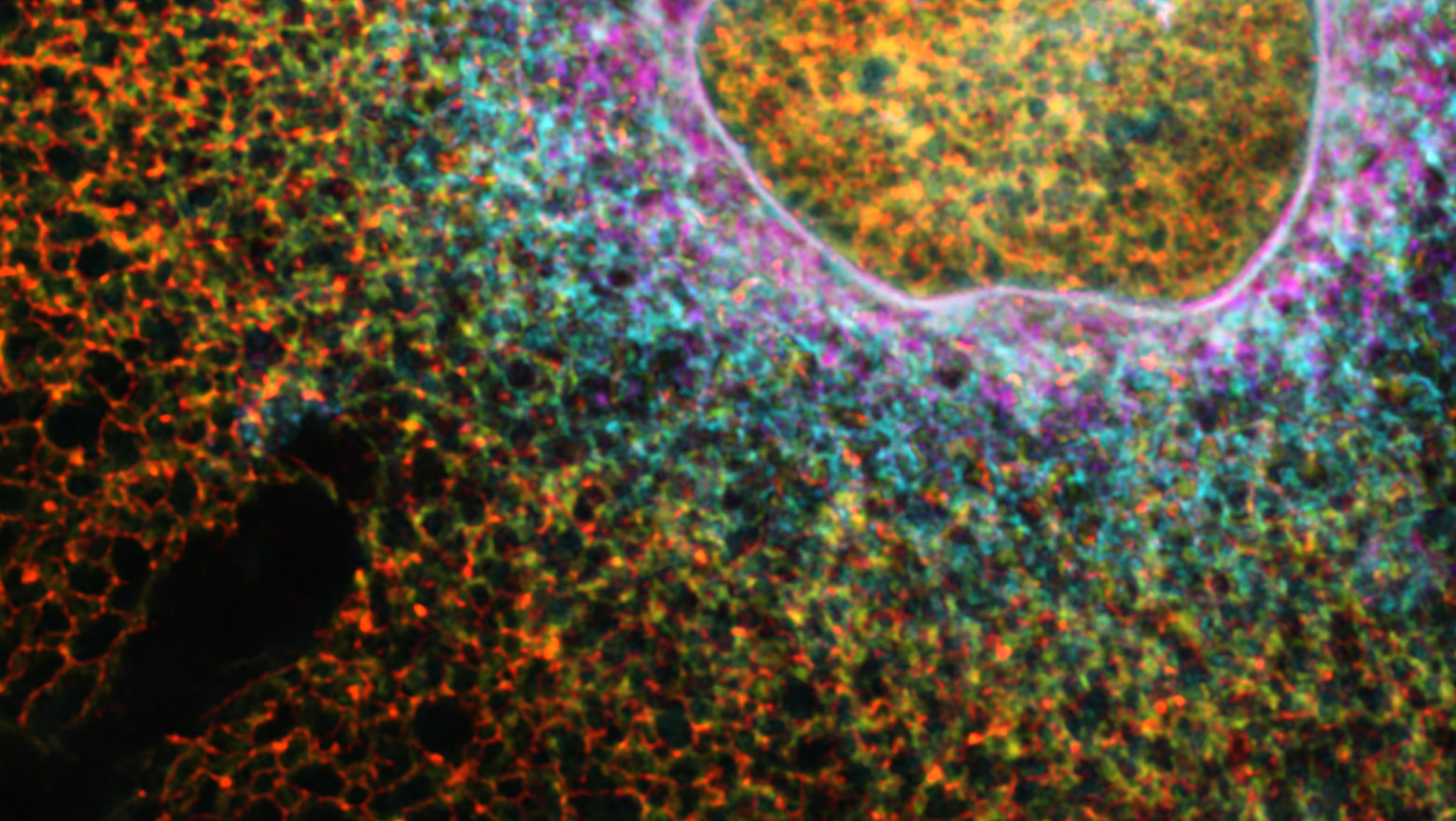
Macropinocytosis regulates cytokine expression through Erk signaling in LPS-stimulated macrophages
この論文ではマクロパイノサイトーシスがErk経路を制御することでサイトカイン発現の調節をしていることを示した。
-
CSF論文発表のお知らせ
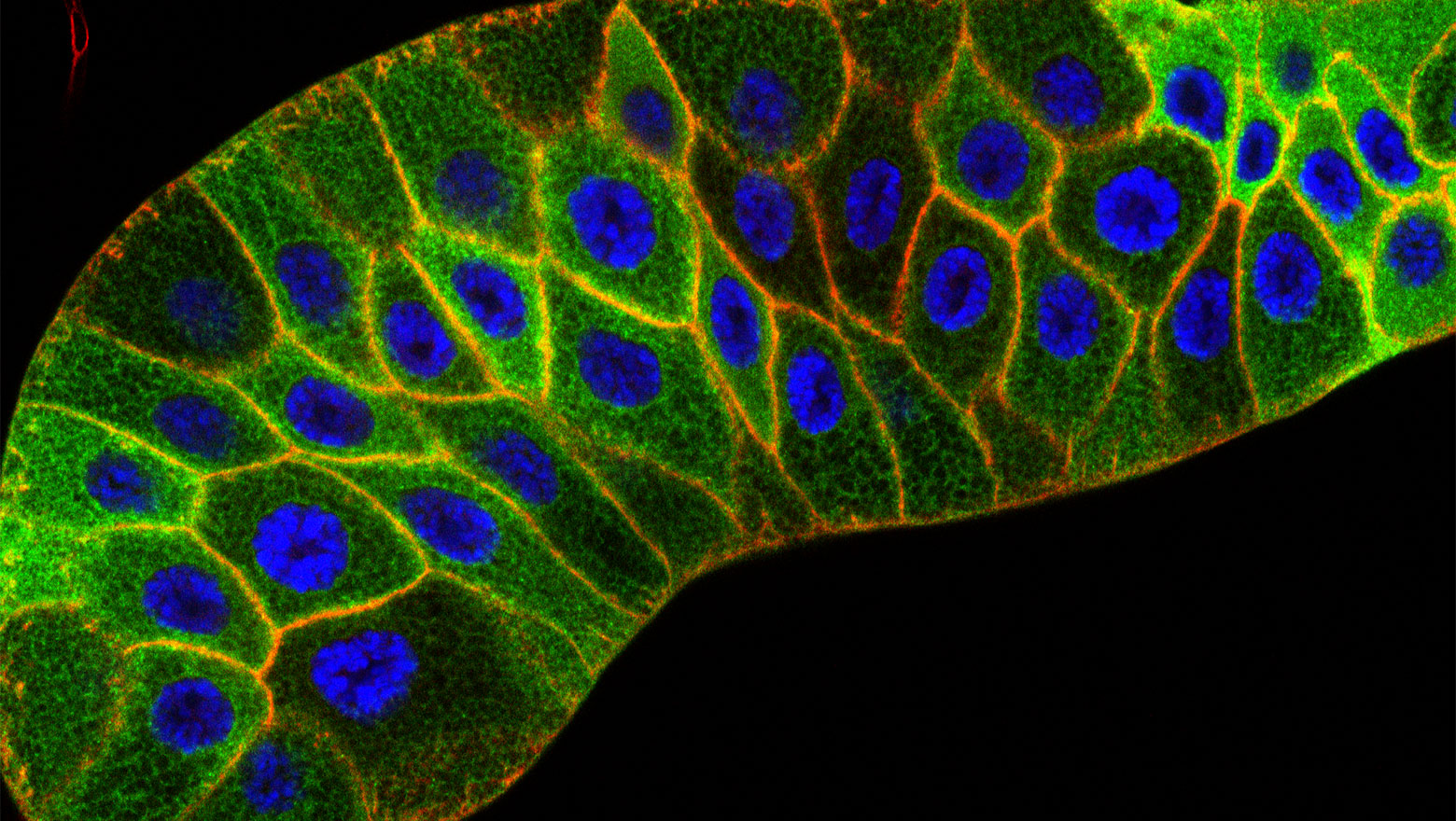
Capturing CDKs in Action: Live-Cell Biosensors Pioneer the New Frontiers in Cell Cycle Research
この論文では、細胞周期を制御するCDKの活性を生細胞でリアルタイムに可視化する蛍光センサーについて概説しています。
-
CSF論文発表のお知らせ
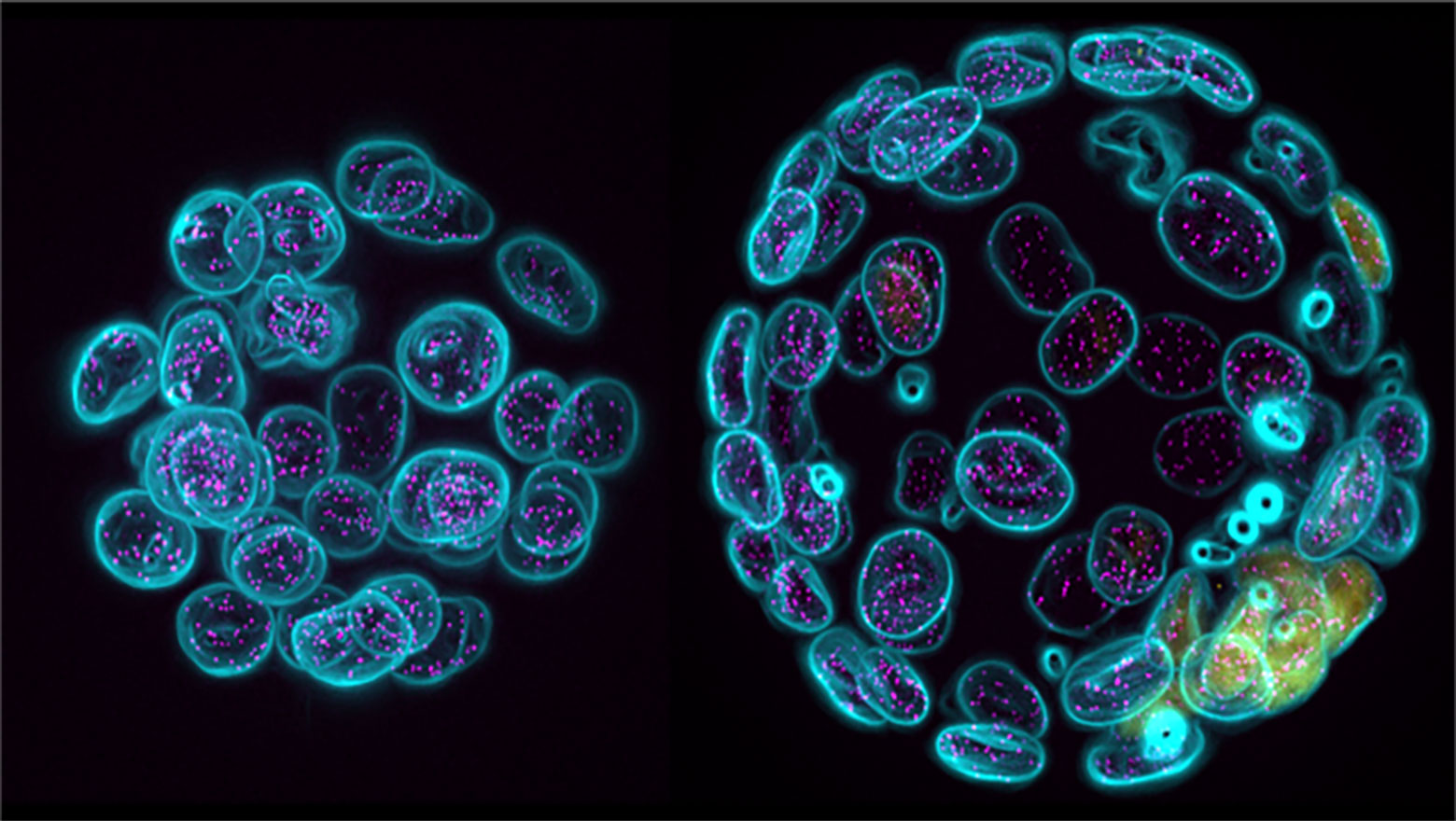
Tango1L but not Tango1S, Tali and cTAGE5 is required for export of type II collagen in medaka fish
この論文は、COPII小胞巨大化に関わる2遺伝子のスプライシング産物4種の中でTango1Lのみがメダカでは必要であることを示した。
Journal
会報「細胞生物」
Events
学術大会・イベント
About
当学会について