生命科学を支える 細胞生物学
News / Topics
新着お知らせ
-
CSF論文発表のお知らせ
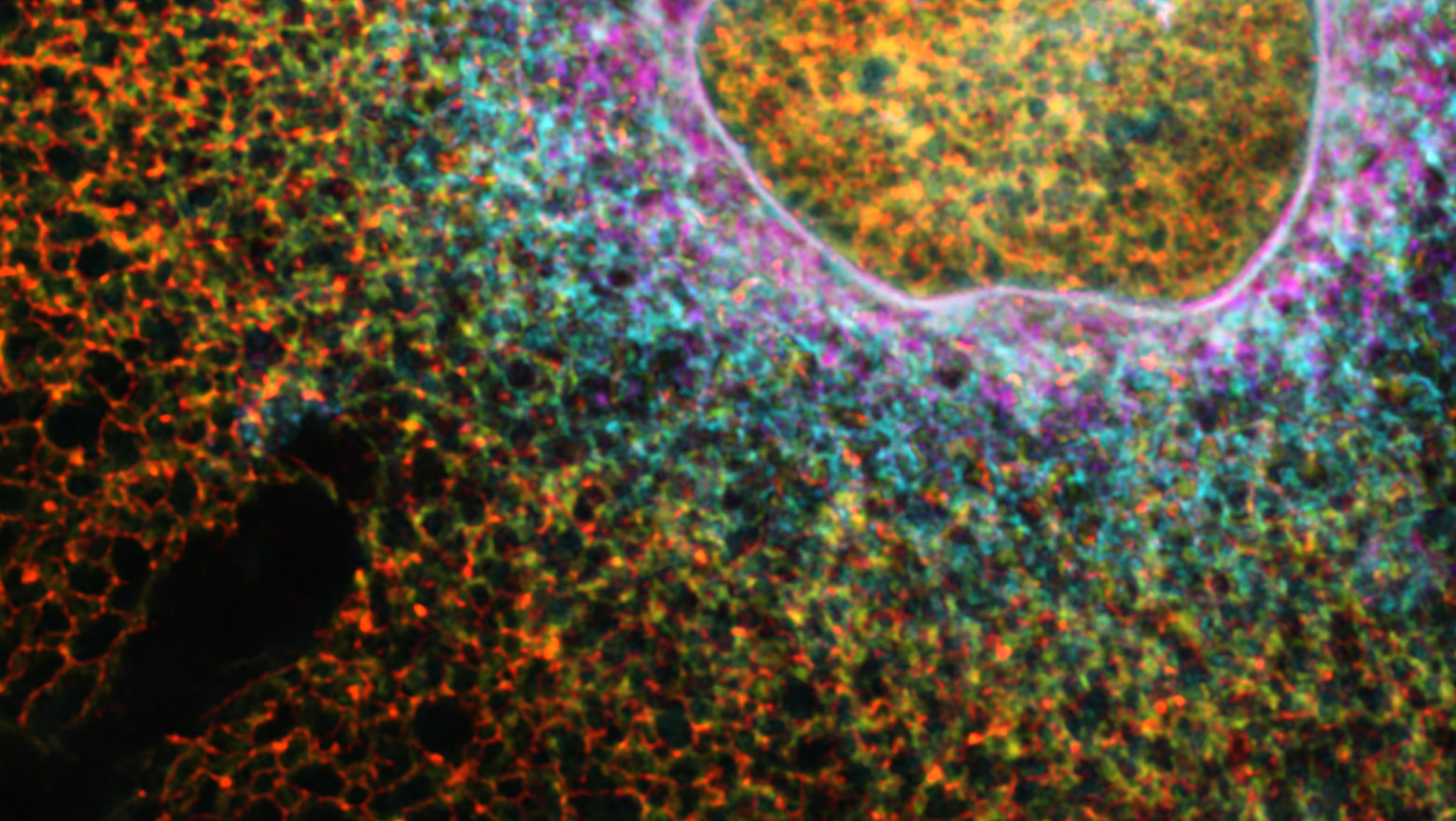
Functional analysis of miR-199a-3p in tumor endothelial cells
腫瘍血管内皮細胞に高発現するmiR-199a-3pがテトラスパニンCD151の発現抑制を介して細胞外マトリックスへの接着低下と遊走能を亢進させた.さらに細胞増殖,浸潤促進をもたらした.miR-199a-3pが腫瘍血管内皮細胞の高い血管新生能の獲得の機序の一部であることが示唆された。
-
CSF論文発表のお知らせ
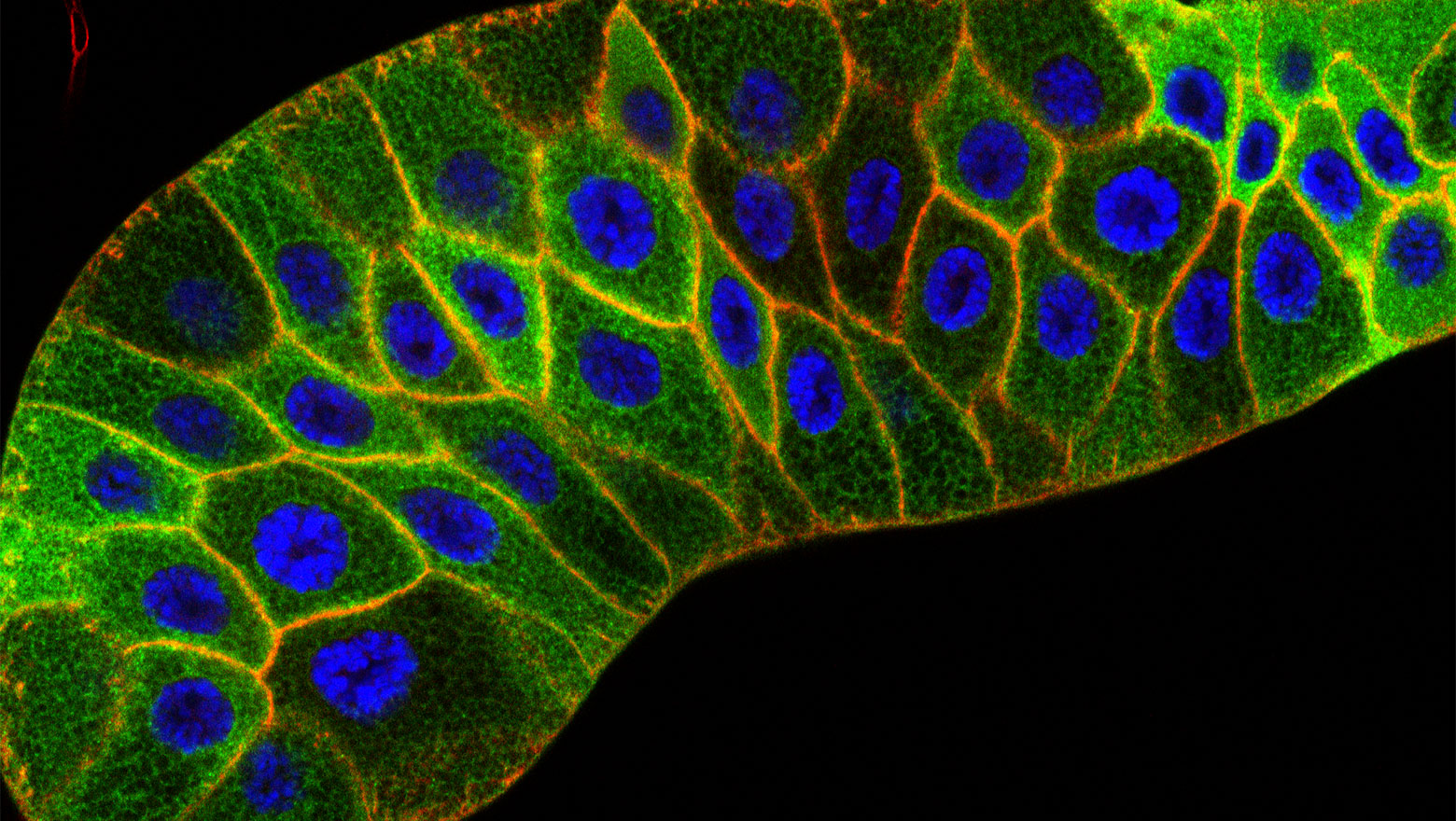
Down-regulation of proliferation-inhibiting factor EGR1 in brain metastatic cancer cells on a soft matrix
本研究は、軟らかい細胞外基質上のメラノーマ高脳転移株では増殖抑制因子であるEGR1の発現が低いことを示しました。
-
CSF論文発表のお知らせ
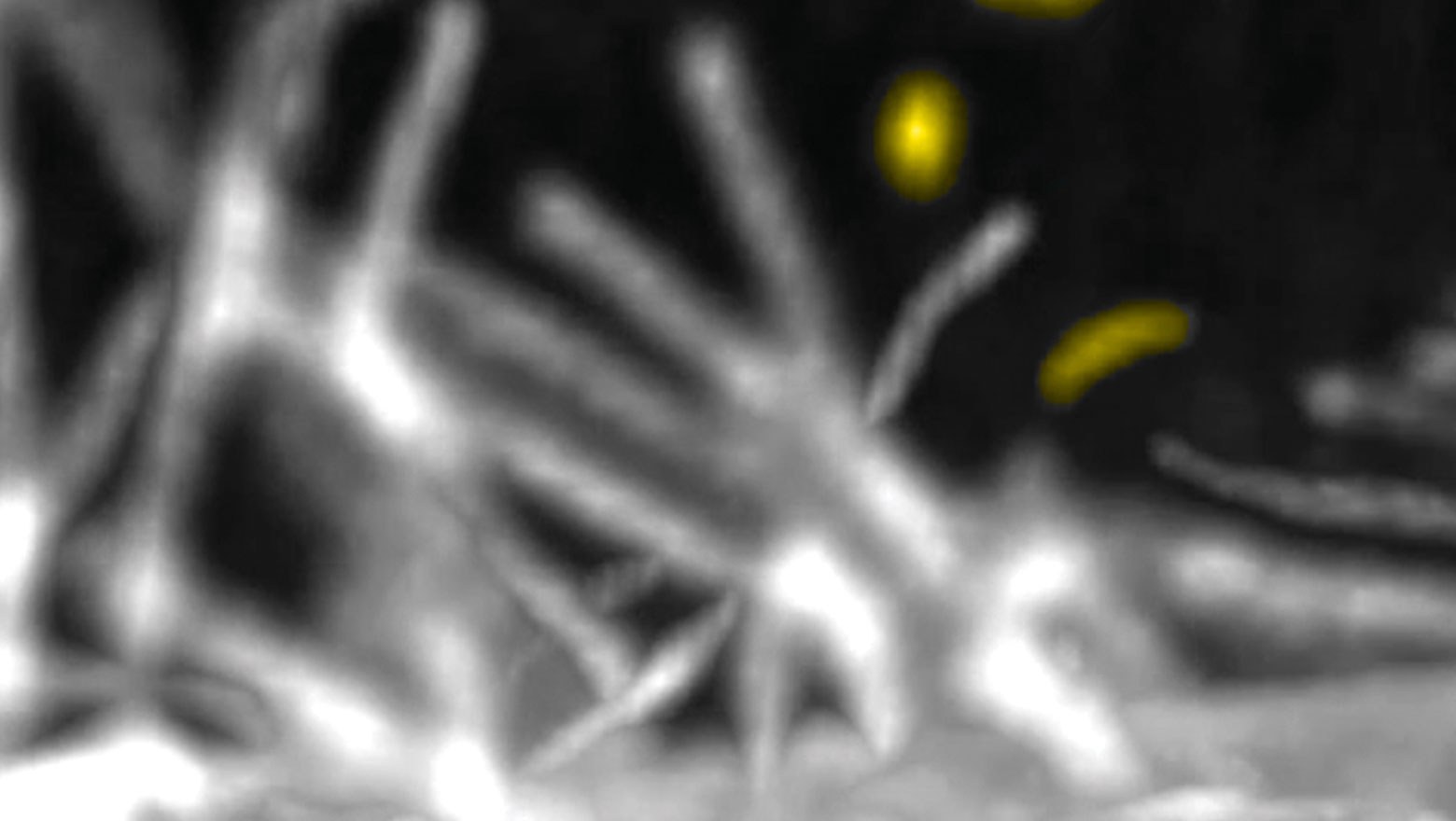
A mei-P26 is required for initiation of meiosis in the Drosophila male germline
TRIM-NHLファミリーに属するショウジョウバエのMei-P26は、雄の減数分裂の開始に必要である。
-
CSF論文発表のお知らせ
Multiplex live imaging approaches to interrogate the interplay of multiple signaling pathways
本論文は、複数の細胞内シグナル伝達経路をライブイメージングで同時に検出する手法(マルチプレックスライブイメージング)を概説したレビュー論文であり、がん研究などへの応用展開の可能性を提示しました。
-
CSF論文発表のお知らせ
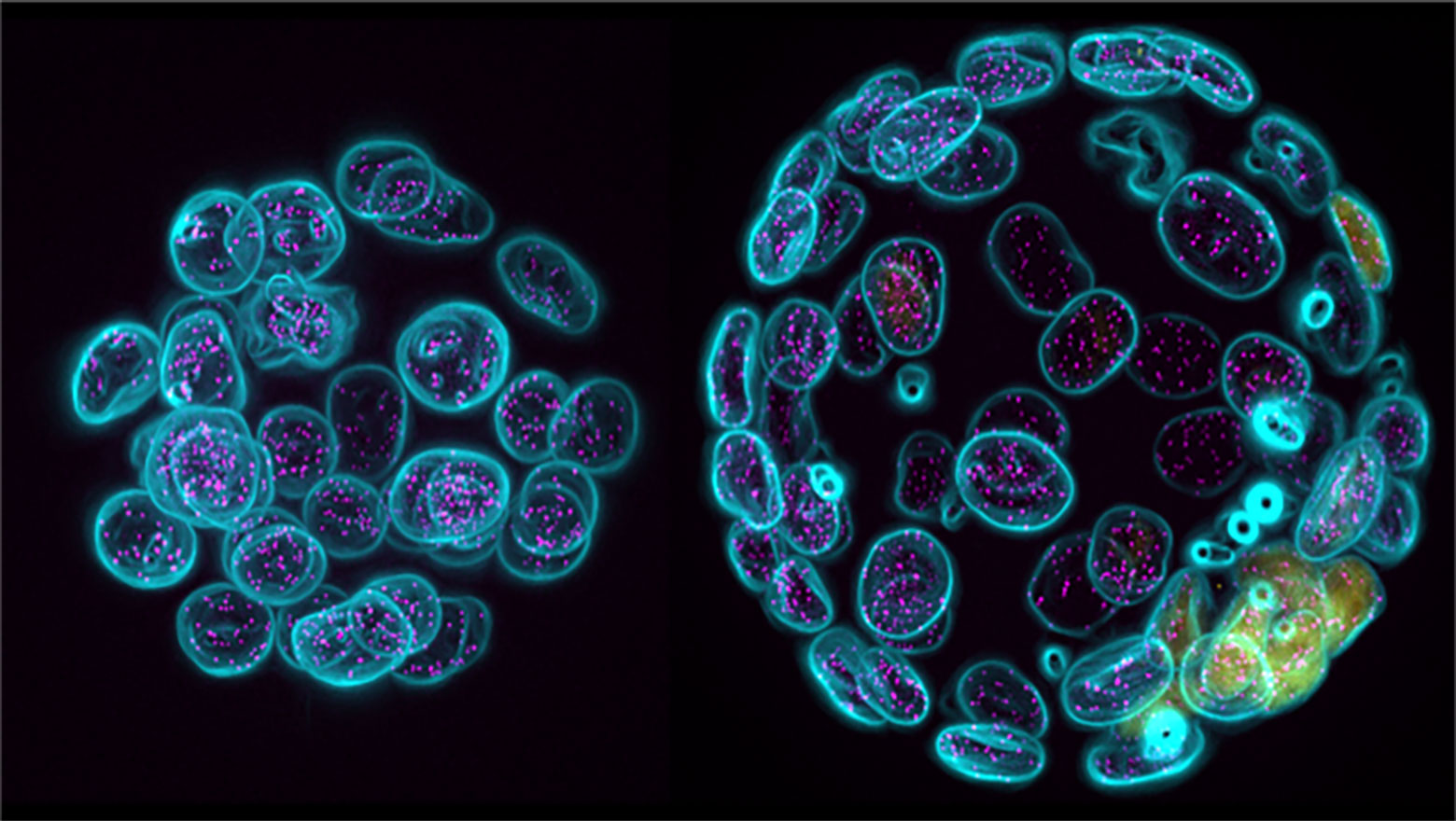
Cholesterol in glioblastoma: impaired Hh signaling enhances epigenetic modifiers and decreases CAV1
This paper explains that high cholesterol and increased CAV1 expression promotes stem-like characteristics in glioma spheroids. Reduction of cholesterol hampers hedgehog signaling and provokes expression of epigenetics modifiers causing epigenetic silencing of CAV1 impacting loss of stem-like phenotypes.
Journal
会報「細胞生物」
Events
学術大会・イベント
About
当学会について